Accreditations & Certifications
Globally Accredited Pharmaceutical Company
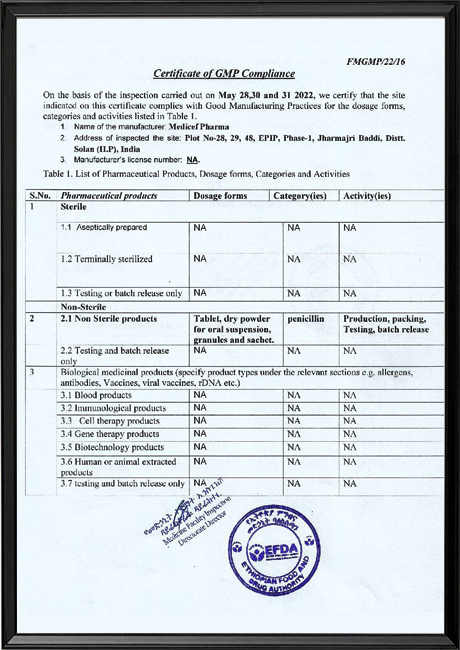
A reputation of consistently delivering high-quality products has helped us become a key partner of choice for multinational companies, Government and Non-Government institutions worldwide. The manufacturing quality has been recognized and as such Medicef is already accredited by multiple health & drug regulatory bodies such as EU-GMP-HUNGARY, MOH-Congo, DPM-Ivory-Coast, FDA-Ghana, MOH-Cambodia, NAFDAC-Nigeria, NDA-Uganda, MOH-Sri-Lanka, MOH-Vietnam and PPB-Kenya amongst others.
Our Certification Goals
Medicef is constantly striving to expand its reach and engage its efforts in regulated as well as semi-regulated markets across the globe. Our immediate goals for the future are, acquiring the following certifications from three of the world’s biggest regions.
Our aim is to penetrate into highly regulated markets such as UK-MHRA, WHO-GENEVA, US-FDA, RUSSIA, COFEPRIS-MEXICO, ANVISA-BRAZIL, TGA-AUSTRALIA while continuously enhancing our Quality Management Systems to meet and exceed the current expectations of regulatory authorities as listed below.

MHRA - UK

FDA - US

RUSSIA

COFEPRIS - MEXICO

ANVISA - BRAZIL

TGA - AUSTRALIA

Corporate Office :
MEDICEF PHARMA
Plot No. 4, Block B, 1st & 2nd Floor, Pocket-8, Near Saroj Hospital, Sector-19, Rohini,
New Delhi - 110089. INDIA.
Group Companies :